SPECIAL TREATMENT
- Saebo
- Balance rehabilitation
- Biodex
- Shockwave therapy
- The Anti-Grav Unweighting System
- Game Ready
- Active passive bike trainer
- Step 400
- Mobility lab
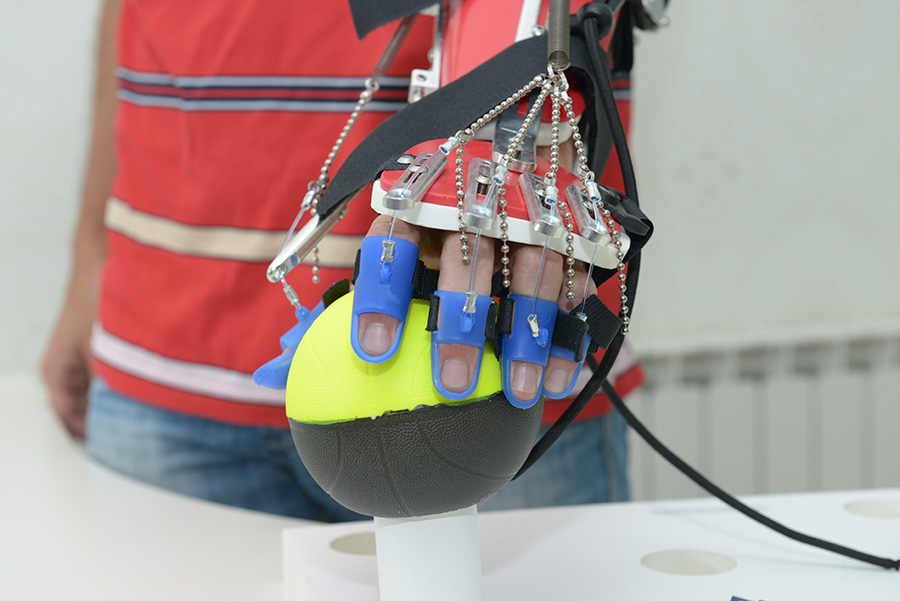
Saebo products enable people with neurological impairments such as stroke the ability to work on their hand function in physiotherapy sessions and at home.
SaeboFlex is an arm training programme designed to help recovery of arm function following an neurological injury. The hand therapy device assists with thumb and finger extension so that grasp and release tasks can be accomplished. The SaeboFlex is suitable for stroke, head injury, spinal injury and cerebral palsy. Clients several years post injury can benefit from the SaeboFlex.
SaeboReach incorporates the affected wrist, hand and elbow. The custom made above elbow cuff allows patients to use their elbow in functional uses, for example, in reaching during grasp and release activities. Along with repetitive task orientated activities the SaeboReach is a highly effective orthosis for maximising arm and hand recovery.
SaeboGlove is a light weight design that helps neurological and orthopedic clients with finger and thumb extension. The SaeboGlove positions the wrist and fingers into extension in preparation for functional activities. The client grasps an object by voluntarily flexing the fingers. The extension system assists with re-opening the hand to release the object.
SaeboStretch uses a revolutionary stretch technology, which allows the fingers to move through flexion caused by associated reactions and tone. The SaeboStretch can overcome issues which can result from traditional splints, namely deformity, joint damage, hypermobility and contractures. It includes three interchangeable hand peices, each with a different grade of resistance. This further protects joints and allows the clinician to customise it for individual needs. The liner is easily removed for routine cleaning
SaeboMAS is a dynamic mobile arm support system with zero gravity, specifically designed to challenge and incorporate the weakened shoulder and elbow during functional tasks and exercises. SaeboMAS enables the patient to perform exercises, as well as self-care activities. It also aids the patient to take part in proven treatment techniques which consist of repetitive tasks which would otherwise have been difficult or impossible. The SaeboMAS is table-mounted, height adjustable, lightweight and portable. It has an elbow support and an adjustable spring base offering various levels of assistance to the user. Multi-directional activities can be achieved
The Balance System has been designed to meet the needs of everyone looking to improve balance, increase agility, develop muscle tone and treat a wide variety of pathologies. Featuring easy-to-follow “touch screen” operation, it is simple to learn and operate, leading the user step-by-step through testing protocols and training modes in both static and dynamic formats. Extremely versatile, it is the only system that provides a fast, accurate Fall Risk Screening and Conditioning Program for older adults; closed-chain, weight-bearing assessment and training for lower extremity patients, and adds the objective balance assessment component to a concussion management program.
Biodex is the world’s first multi-mode computerized robotic Dynamometer.
Sports and orthopedic medicine, pediatric medicine, neurorehabilitation, older adult medicine, industrial medicine, and researchers depend on Biodex to provide consistent, accurate, objective data. Objective data that provides the best outcome for their patients… objective data that supports their research… objective data that separates their facility from the rest.
Biodex Objective Data helps you communicate need, progress and outcome clearly and accurately. Easy to read and interpret color graphic reports are produced with normative data. Graphic reports and narrative letters compare patient’s status to normative data for all joints. Data legends and on-screen editing helps communicate the information in simple terms for patients, doctors, third party payers and employers.
Shockwave therapy is a multidisciplinary device used in orthopedics, physiotherapy, sports medicine, urology and veterinary medicine. Its main assets are fast pain relief and mobility restoration. Together with being a non-surgical therapy with no need for painkillers makes it an ideal therapy to speed up recovery and cure various indications causing acute or chronic pain.
Shockwave is an acoustic wave which carries high energy to painful spots and myoskeletal tissues with sub acute, sub chronic and chronic conditions. The energy promotes regeneration and reparative processes of the bones, tendons and other soft tissues.
Painful Heel is a common syndrome characterized by severe pain in the inferior or posterior aspect of the heel, which is aggravated by weight bearing, becoming progressively worse and often incapacitating, with evidence of a spur in about 50% of cases.
Until now the cause of the condition has been obscure, but numerous factors have been claimed to produce painful heel with a bony spur: functional overuse, degenerative diseases, inflammatory diseases, and metabolic diseases.
The conservative methods of treatment usually adopted have included insole supports, injections of local anesthesia and corticosteroids, and medical treatment
Extracorporeal shock wave treatment (ESWT) is based on the use of shock waves, that is, microsecond pressure impulses, which, depending on the energy used, can reduce painful symptoms and fragmentation of calcific deposits
We evaluate, in our outpatient department, the efficacy of the extracorporeal shock wave treatment (ESWT) in calcaneal enthesophytosis. Our Patients, had talalgia associated with heel spur, are diagnosed and referred by a medical prescription.
After 6 sessions of ESWT, calcaneal enthesophytosis examination was made by x and Pain levels were evaluated by a visual analogue scale (VAS).
X ray showed morphological modifications (reduction of the larger diameter) of the enthesophytosis and decrease of VAS.
ESWT is safe and improves the symptoms of most patients with a painful heel, it can also structurally modify enthesophytosis, and reduce inflammatory oedema.
The Anti-Grav Unweighting System allows an individual to reduce their overall body weight during physical activity. We have successfully implemented this system to fit the needs of general physical therapy, post-surgical recovery, elderly exercise assistance, obese patient exercise, and elite athlete training.
The Anti-Grav Unweighting Machine allows therapists and trainers to provide a streamlined evaluation and targeted rehabilitation process. This allows users and therapists to diagnose and correct errors in movement and form while walking, running, and other various weight bearing activities. This is accomplished with greater accuracy when the user is in an unloaded state, allowing superior visualization of gait and movement mechanics without pain.
Our system allows an extremely low stress and total customizable variable load resistance to help in aiding the recovery process. We believe the AGU System can help millions of people to regain health, live a fuller life, and redeem their self-confidence.
Game Ready re-engineers recovery with leading technologies inspired by human endeavor – all designed to get people back to being their best after injury or surgery. Thousands of patients, elite athletes, athletic trainers, pro teams in every sport, special military forces, and the world’s foremost orthopedic surgeons, sports medicine doctors, and physical therapists choose Game Ready for our commitment to developing the world’s most innovative, most effective recovery products.
The Active passive bike Trainer is the ideal exercise therapy equipment for many users with different conditions. With an Active and Passive lower and upper body trainer, a safety handle, calf rests and 2.7” color touch screen, it is the perfect exercise therapy equipment for conditions such as Multiple Sclerosis, Spinal Injury, Muscle Dystrophy, Stroke, Head Injury and other neurological conditions.
The Step 400 is a battery-operated, functional electrical stimulator (FES) that helps reduce foot drop. Central nervous system injuries often cause a gait disorder called ‘Foot Drop’, which is the inability to raise the foot while walking, therefore resulting in dragging of the foot, instability and increased effort during gait.
Electrical stimulation has been used safely in rehabilitation for decades. There is an abundant amount of research supporting the use of FES as a treatment option for foot drop. Unfortunately, it has been inaccessible due to the bulkiness of the equipment and the grave costs.
The Step 400 is a modern FES device that delivers safe, low-level electrical pulses to the peroneal nerve which controls foot drop. The Step 400 intelligently raises the foot at the appropriate phase of walking, therefore, increasing patient safety, speed and confidence when walking!
Unique to the STEP 400 are it’s 2 set up configurations including a Cuff design which ensures constant and snug contact between the user’s limb and integrated electrodes. Additionally, the user has the option to remove the cuff from the stimulator allowing for a low profile belt clip attachment. Information regarding the stimulator’s connection status and program configuration are displayed vividly on the OLED screen.
Developed by expert Physical Therapists the Step 400 was designed for quick set up, simple operation, reliability and affordability.
Clinical Application
Millions of patients worldwide suffer from foot drop. Foot Drop is a medical condition where your foot drags as you walk, making it difficult to walk quickly and safely. The Step 400 uses small carefully calibrated electrical currents to activate the muscles and nerves in the leg and foot in order to lift your foot and allow you to walk more easily.
The Step 400 may help individuals who have the following conditions:
· ALS
· Stroke
· Traumatic brain injury
· Incomplete spinal cord injury
· Multiple sclerosis
· Cerebral Palsy
· Post Polio
Patient Benefits:
The benefits of using gait training can be categorized into three main domains: clinical outcomes, fitness benefits and functional gains.
Clinical:
· Improve muscle tone/mass
· Muscle reeducation
· Increase range of motion in the foot/ankle.
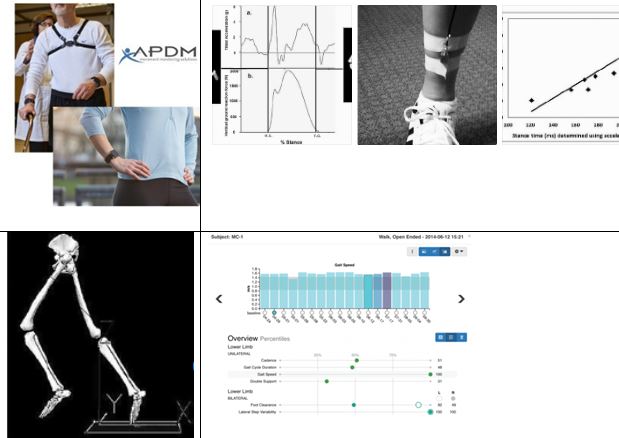
Mobility Lab™ is the first portable gait and balance laboratory designed for clinicians and clinical researchers. It was designed to streamline gait and balance assessment by making it easy to collect, store, analyze, and interpret data.
Mobility Lab™ is composed of:
1) a set wireless, body-worn Opal™ inertial sensors, each with a docking station,
2) an Access Point for wireless data transmission and sub-millisecond synchronization of the independent sensors,
3) user-friendly software to guide the user and subject(s) through the testing protocols, and
4) automated analysis and reporting of the recorded data.
Depending on the specified test protocol, one to six Opals are attached to the body with Velcro straps (one on the low back for postural sway, two on the shanks for gait, one on the sternum for sit/stand transitions, and two on the wrists for arm swing). shows a subject wearing the Opals while performing a 180-degree turn. While being guided through the protocol, the clinician can easily abort, repeat, or comment on trials as necessary. Mobility Lab™ provides immediate access to measures of gait and balance along with matching control values to aid in research or clinical decision-making.
Mobility lab plugins:
APDM offers a suite of plugins for Mobility Lab™ that allows clinicians to customize their analytical software to satisfy their research, clinical or therapeutic needs. Each plugin is an instrumented version of a widely-accepted clinical protocol.
Instrumented Timed-up and go plugin (ITUG): Subjects are instructed to stand up from a chair, walk 7 m, turn 180°, then walk back and sit down. The distance walked was extended from 3 to 7 m to allow processing of gait cycle data. The ITUG objectively characterizes 53 parameters during postural transitions (sit-to-stand and turn-to-sit), walking and turning. Each of these parameters has been previously validated with a motion analysis system in a gait laboratory
Instrumented Sway (ISway): subjects are instructed to stand with arms at their side. The size of their stance is fixed with a spacer block placed between the feet. ISway objectively measures amplitude, velocity, frequency and jerkiness of postural sway in both the lateral and anterior-posterior directions with 42 metrics. Each of these metrics have been validated against postural sway measured from center of pressure displacement with a force place. Therapists interested in performing the Clinical Test of Sensory Integration for Balance (CTSIB), measure sway while patients stand either on a firm surface or on compliant foam with eyes opened or closed to evaluate use of surface, visual, and vestibular information for postural sway
Instrumented Stand and Walk (ISAW): This short test was designed to combine measures of postural sway, anticipatory postural adjustments during step initiation, gait and turning into one, quick protocol. Subjects are instructed to stand quietly for 30s and then asked to walk at their comfortable speed for 7m, turn 180° and walk back to the starting point summarizes the subcomponents of mobility and type of metrics calculated for the ISAW.
Instrumented Long Walk plugin (IWalk): This is the only plugin that doesn’t have a fixed protocol since patients can walk any distance from 7m up to 7 km as long as all of the walking is on a straight path and all of the turns are 180 degrees. IWalk allows additional gait parameters to be calculated that cannot otherwise be measured with short walking distances, such as gait variability, coordination (phase coordination index), and asymmetry
Mobility Lab Reporting
The report shows many type of measures calculated in Mobility Lab from a PD patient undergoing deep brain stimulation (DBS). In this example, the ITUG and ISWAY were performed after six different stimulation settings. It is interesting to see that the 3rd setting showed a worsening in balance and transitions but not in gait. In fact, sit-to-stand duration and turn duration were the worst in the 3rd DBS setting, while stride length and stride velocity are consistent across all settings. Also, sway jerk and sway frequency were the worst in that particular setting. A patient with this particular DBS setting would need therapy for postural transitions and balance, but not for gait itself
Sensitivity, reliability, and validity of many of the plugins have been previously reported. In particular, lateral postural sway, trunk rotation during gait, and arm swing during gait and turning are very sensitive to mild disease, such as mild-to-moderate untreated PD and multiple sclerosis. In fact, postural sway, anticipatory postural adjustments, arm speed, and turning velocity are sensitive measures in early PD patients who otherwise have normal Timed Up and Go times and gait speed. Test-retest reliability, after taking the sensors off and re-applying them can be excellent (ICC ranged from .75 to .98), with help from algorithms that compensate for inconsistent sensor placement Concurrent validity of the ITUG and ISway have been established with popular clinical tests of balance and gait such as the posture and gait part (PIGD) of the UPDRS.
